- Orchid Brand Launch – PPE & Medical Devices
- Orchid PPE & MD – Glossary and Reference
ORCHID – a new brand
AN INTRODUCTION TO GLOVE CERTIFICATION & PACKAGING SYMBOLS
SYMBOL
DESCRIPTION
OFFICIAL RESOURCE
CORPORATE – including product

Conformité Européenne
Certificate of Conformity to European Standards Required for all medical devices.

UK Conformity Assessment
In-process UK replacement to CE & MD / MDR.

ISO 9001:2015
Manufacturing Quality Management System (QMS).

ISO 13485:2016
Medical Devices Manufacturing Quality Management System (QMS).

EC REP (for Medical Devices)
EC Representative Information; denotes that an EC representative is appointed and responsible for EU registrations.
PRODUCT SPECIFIC CERTIFICATIONS / SYMBOLS

REACH Compliant
Regulation (EC) No 1907/2006 – Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH).
Denotes that the product has been tested for the more than 200 substances not permitted to be used in products sold in the EU.

MD is a standard used globally to mark a Medical Device. In Europe as an MDR, Registered and authorised for use as a Medical Device in the EEA/EC/EU.
For the UK, see UKCA information.

FDA / FDA 510K
Registered and authorised for use in the USA by the Food and Drug Agency.

PPE Category III (3).
Denotes the product has passed all tests and obtained all registrations to be classified as PPE Category 3.
Other Categories 1 and 2 are not to the same standard.
N/A

ISO 374
Protective gloves against dangerous chemicals and micro-organisms.
Constitutes four parts, detailed below.

ISO-EN-374-1
Protective gloves against dangerous chemicals and micro-organisms.
Letters denote which chemicals were used in testing.
Type A – min. 6 Chemicals >= 30 minutes
Type B – min. 3 Chemicals >= 30 minutes
Type C – min. 1 Chemicals >= 10 minutes

EN ISO 374-2:2019
Resistance to Penetration, protect against dangerous chemicals and/or micro-organisms.
Air Leak & Water Leak.

EN ISO 374-4
Protective Gloves – Degradation and resistance to chemicals;
and
ASTM D6978-5
Permeation by Chemotherapy Drugs.
The symbol is not a standard for these, but often used to denote the same.


EN ISO 374-5:2016
Protective gloves – against bacteria and fungi, also viruses.
The second symbol denotes part 5.3 was also tested, beyond parts 5.1 and 5.2.

EN455
Constitutes four parts required to determine the medical requirements beyond PPE.
EN 455-1 Freedom from holes.
EN 455-2 Physical properties.
EN 455-3 Biological Evaluation; freedom from residual powder.
EN 455-4 Shelf Life – Meets performance after 3 years of ageing.
EN 455 – Source Satra

ASTM D5151
Detection of Holes in Medical Gloves.

ASTM D6124
Detection of Residual powder.

ASTM D6319
Medical Application Nitrile Gloves.

ASTM D6978-5
Permeation by Chemotherapy Drugs.

ASTM F1671
Resistance of Materials Used in Protective Clothing to Penetration by Blood-Borne Pathogens.

AQL 1.5
“Acceptable Quality Level” – the minimum standard for Medical Gloves.
Note: Patient Examination Gloves have an AQL of 2.5; i.e. a much higher defect rate allied with less certifications to meet the grades required today.

BS-EN-1186 – Materials and articles in contact with foodstuffs.
Safe for use when handling food and drink.

Non-Sterile Gloves Symbol.
N/A

Single Use item.
Once used / touched dispose of and do not reuse.
N/A

Powder Free
The product is tested for residual powder and meets the minimum standard per EN455-3 and ASTM D6124.

Contains no Latex.
The product has been tested to confirm that it is Latex-Free.
N/A
STORAGE & HANDLING

Date of Manufacture
N/A

Use by Date /
Expiry Date
N/A

Fragile Contents
Handling instructions must be observed
N/A
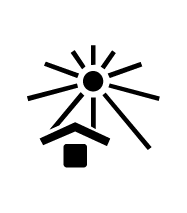
Keep out of Direct Sunlight
N/A

Keep Dry / Keep out of Rain
N/A

Caution: Consult Accompanying Documentation
N/A

Instructions for Use
N/A

LOT / Batch Number
N/A
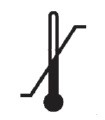
Temperature Limits for Storage
N/A

Manufacturer
N/A
ORCHID
a new brand
AN INTRODUCTION TO GLOVE CERTIFICATION & PACKAGING SYMBOLS
SYMBOL
DESCRIPTION
OFFICIAL RESOURCE
CORPORATE – including product

Conformité Européenne
Certificate of Conformity to European Standards.

ISO 9001:2015
Manufacturing Quality Management System (QMS).
UK Conformity Assessment
In-process UK replacement to CE & MD / MDR.


ISO 13485:2016
Medical Devices Manufacturing Quality Management System (QMS).

EC REP (for Medical Devices)
EC Representative Information; denotes that an EC representative is appointed and responsible for EU registrations.
PRODUCT SPECIFIC CERTIFICATIONS / SYMBOLS

REACH Compliant
Regulation (EC) No 1907/2006 – Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH).
Denotes that the product has been tested for the more than 200 substances not permitted to be used in products sold in the EU.

MD is a standard used globally to mark a Medical Device. In Europe as an MDR, Registered and authorised for use as a Medical Device in the EEA/EC/EU.
For the UK, see UKCA information.

FDA / FDA 510K
Registered and authorised for use in the USA by the Food and Drug Agency.

PPE Category III (3).
Denotes the product has passed all tests and obtained all registrations to be classified as PPE Category 3.
Other Categories 1 and 2 are not to the same standard.
N/A

ISO 374
Protective gloves against dangerous chemicals and micro-organisms.
Constitutes four parts, detailed below.

ISO-EN-374-1
Protective gloves against dangerous chemicals and micro-organisms.
Letters denote which chemicals were used in testing.
Type A – min. 6 Chemicals >= 30 minutes
Type B – min. 3 Chemicals >= 30 minutes
Type C – min. 1 Chemicals >= 10 minutes

EN ISO 374-2:2019
Resistance to Penetration, protect against dangerous chemicals and/or micro-organisms.
Air Leak & Water Leak.

EN ISO 374-4
Protective Gloves – Degradation and resistance to chemicals;
and
ASTM D6978-5
Permeation by Chemotherapy Drugs.
The symbol is not a standard for these, but often used to denote the same.


EN ISO 374-5:2016
Protective gloves – against bacteria and fungi, also viruses.
The second symbol denotes part 5.3 was also tested, beyond parts 5.1 and 5.2.

EN455
Constitutes four parts required to determine the medical requirements beyond PPE.
EN 455-1 Freedom from holes.
EN 455-2 Physical properties.
EN 455-3 Biological Evaluation; freedom from residual powder.
EN 455-4 Shelf Life – Meets performance after 3 years of ageing.
EN 455 – Source Satra

ASTM D5151
Detection of Holes in Medical Gloves.

ASTM D6124
Detection of Residual powder.

ASTM D6319
Medical Application Nitrile Gloves.


ASTM D6978-5
Permeation by Chemotherapy Drugs.
ASTM F1671
Resistance of Materials Used in Protective Clothing to Penetration by Blood-Borne Pathogens.

AQL 1.5
“Acceptable Quality Level” – the minimum standard for Medical Gloves.
Note: Patient Examination Gloves have an AQL of 2.5; i.e. a much higher defect rate allied with less certifications to meet the grades required today.

BS-EN-1186 – Materials and articles in contact with foodstuffs.
Safe for use when handling food and drink.

Non-Sterile Gloves Symbol.
N/A

Single Use item.
Once used / touched dispose of and do not reuse.
N/A

Powder Free
The product is tested for residual powder and meets the minimum standard per EN455-3 and ASTM D6124.

Contains no Latex.
The product has been tested to confirm that it is Latex-Free
N/A
STORAGE & HANDLING

Date of Manufacture
N/A

Use by Date /
Expiry Date
N/A

Fragile Contents
Handling instructions must be observed
N/A

Keep out of Direct Sunlight
N/A

Keep Dry / Keep out of Rain
N/A

Caution: Consult Accompanying Documentation
N/A

Instructions for Use
N/A

LOT / Batch Number
N/A

Temperature Limits for Storage
N/A

N/A
Manufacturer
We hope you found this resource useful.
Please contact us if you would like to learn more or request product quotations for any of our offerings or services.

